Get Latest Updates About Pharmacy Notes, Books and Many More
Aim: To prepare and submit Tolbutamide from Toluene
Reference: Jayaveera K.N., Subramanyam S., Reddy K.Y., Practical Medicinal Chemistry: S.Chand Publication; 2018, Vol. 53; Page no 397
Chemicals Required: Concentratic Sulphuric acid, Toluene, Phosphorus pentachloride, Iodine Lime water, Sodium Carbonate, Ethyle chloroformate, Ammonium carbonate, Anhydrous potassium Carbonate, Acetic acid, dry acetone, Butyl amine, Butylisocynate
Apparatus Required: RBE, Condenser, Beaker, Funnel, and Glass rod
Principles: Tolbutamide, NP-methylbenezenesuphonyl)-N-butyl urea is an oral hyperglycemic
agent used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM). It is structurally similar to acetohexamide, chlorpropamide, and tolazamide and belongs to the sulphonylurea class
of insulin secretagogues which act by stimulating the beta cells of the pancreas to release insulin.
Sulphonylureas increase both basal insulin secretion and metal stimulated insulin release
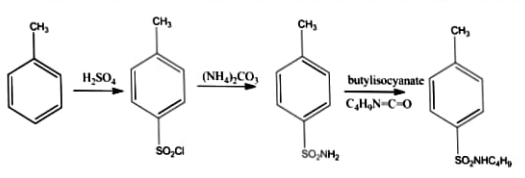
Procedure:
Procedure for synthesis of p-toluenesulphonyl chloride from toluene
- Heat 130 gm of pure toluene and 450 gm conc. sulphuric acid in a cast- iron pot fitted with suitable agitator. Add few crystals of iodine and allow the temperature to rise 100c.
- The sulphonation is complete in about 6 hrs, transfer the reaction mixture in a large beaker diluted with water. Add lime water gradually neutralize the excess acid.
- Filter out the calcium sulfate and any ferric hydroxide and wash with hot water.
- Add sodium carbonate to the filtrate until just alkaline to phenolphthalein and remove the calcium carbonate by filtration. Evaporate the filtrate almost to dryness, when sodium salt of o toluene Sulphonyl and p-toluene sulphonyl acids separate out. When the reaction is complete add cold water. The p-toluene sulphonyl chloride separates as a solid and recrystallizes with alcohol. The O-toluene sulphonyl chloride is an oily liquid and is separated from the filtrate by means of a separating funnel.
Procedure for synthesis of p-toluene sulphonamide
- Thoroughly mix 5 grams of p-1oluene sulphonyl chloride and 10 grams of ammonium carbonate by grinding in a mortar until a fine powder is obtained.
- Heat the mixture on a water bath with continuous stirring to convert sulphonyl chloride into sulphonamide. Crystallize the product from hot water. Filter off as colourless crystals.
Procedure for the synthesis of ethyl p-toluene sulphonyl carbamate
- Mix the corresponding sulphonamide 49 grams with ethyl chloroformate 42 grams and anhydrous potassium carbonate 3 grams in dry acetone 400 ml and reflux for 20 hours. Remove acetone by distillation under reduced pressure and keep the solution overnight and then add in water 250 ml. Neutralize with acetic acid. Filter the solid product and wash with distilled water and recrystallize with alcohol.
Procedure for synthesis of tolbutamide
- Dissolve the specified carbamate derivative in hot toluene 30 ml. Add butylamine slowly and reflux for 4 hours. Filter and dry it.
Report: Tolbutamide was prepared and submitted.
Uses: Tolbutamide is used together with diet and exercise to improve blood sugar control in adults
with type 2 diabetes mellitus. Tolbutamide is not for treating type I diabetes.