Get Latest Updates About Pharmacy Notes, Books and Many More
Aim
To prepare and submit hexamine from formaldehyde and calculate its Percentage Yield.
Principle
Hexamine is a heterocyclic organic compound (CH2)6N4. It has a symmetrical tetrahedral cage-like structure. It is prepared by a condensation
reaction between formaldehyde and ammonia.
Reaction
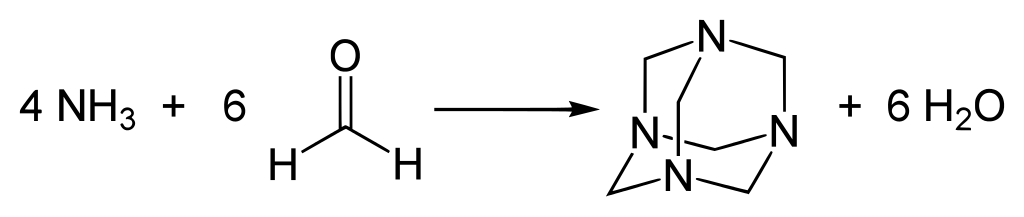
Requirements
- Formaldehyde – 4.7 g
- Ammonia Solution – 7 g
Procedure
About 4.7g of 30% formaldehyde solution was taken in a beaker and add 7g of 24% ammonia solution was until the solution is slightly alkaline. The mixture was heated on a water bath for 5 minutes and allowed to stand for 15 minutes. The solution was filtered and then evaporated on a direct flame using a china dish to a thick paste. The hexamine crystals are obtained and dried. It was recrystallized from water or alcohol. Hexamine forms colourless, odourless crystals, which are soluble in water and 90% alcohol.
Use: Urinary anti-infective agent
Report
Hexamine was prepared and submitted. Report the following
Theoretical Yield:
Practical Yield:
Percentage Yield: