Get Latest Updates About Pharmacy Notes, Books and Many More
Aim
To determine the percentage purity of a given sample of Isonicotinic acid hydrazide tablet.
Principle
Isonicotinic acid hydrazide/ isoniazid is an anti-tubercular drug. It is assayed by the direct titration of Potassium bromate with the addition of potassium bromide in the presence of acid medium using hydrochloric acid. During oxidation reaction liberated bromine reacts with isoniazid in an aqueous solution to form isonicotinic acid. Azo dye methyl red indicator is used in this titration, decolourized the red colour is the end point.
Reaction
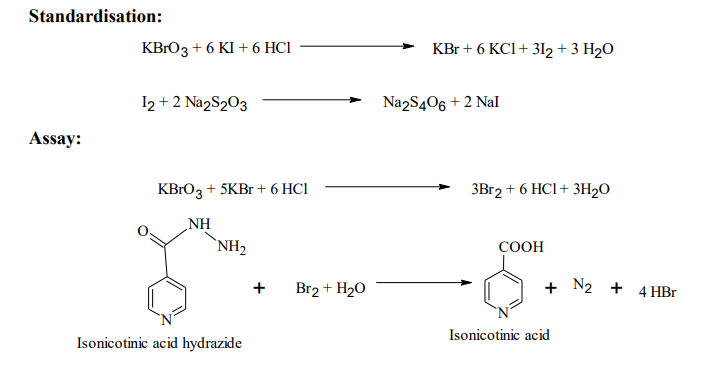
Procedure
Preparation of 0.0167 M Potassium bromate solution
About 5.566gm of Potassium bromate was dissolved in water and made up to 1000 ml with distilled water.
Standardisation of 0.0167 M Potassium bromate
20 ml of the above solution was transferred in a glass stopper flask and 3 g of Potassium iodide and followed with 3 ml of HCl was added. Allow standing for 5 min, titrate liberated Iodine with 0.1 M sodium thiosulphate adding 3 ml of starch solution TS and the endpoint is approached.
The concentration of 0.0167 M Potassium bromate = (Molarity of Sodium thiosulphate)/(Volume of Potassium bromate)
Assay of Isonicotinic acid hydrazide tablet
Twenty tablets were weighed accurately and pulverized. A weighed quantity of the tablet power equivalent to 0.25 mg INH was transferred into a
clean and dry 100 ml volumetric flask, then sufficient water was added to produce 100 ml. 20 ml of the above solution was taken. Then 100 ml of water, 20 ml of hydrochloric acid and 0.2 gm of Potassium bromide were added. Then titrated slowly with continuous shaking with 0.0167M potassium bromate using 0.05ml of methyl red as an indicator until the red colour disappears.
Each ml of 0.0167M Potassium bromate KBrO3 = 0.003439g of C6H7N3O.
Report
The Molarity of 0.1 M Sodium nitrite
The percentage purity of the given isonicotinic acid Hydrazide tablet was found to be: ______________________